The Promise and Challenges of Regulatory T Cell Therapy: A New Frontier in Immunotherapy
In recent years, regulatory T cell (Treg) therapy has emerged as a ground breaking approach to cellular immunotherapy. Treg therapies hold immense potential to combat autoimmune diseases, prevent transplant rejection, and treat inflammatory conditions by harnessing the body’s natural immune regulators. However, Treg therapies bring an additional layer of complexity compared to other adoptive cell therapies, such as CAR-T. While all cell therapy manufacturing is challenging, the rarity of Tregs, coupled with the critical need for a highly pure cell population, makes their development and production even more demanding.
Understanding Regulatory T Cells: Nature’s Immune Regulators
Regulatory T cells (Tregs) are specialized immune cells that act as regulators of inflammatory responses. A subset of CD4+ T cells (5-7%), they are characterized by high expression of the transcription factor FOXP3 and can be identified and isolated for therapeutic purposes using the unique combination of CD4+CD25+ high and CD127 low cell surface markers.
Tregs regulate immune responses through several mechanisms, including:
-
- Production of anti-inflammatory cytokines: Such as IL-10, IL-35, and TGF-β.
-
- Consumption of pro-inflammatory cytokines: Like IL-2.
-
- Suppression of immune cells: Via regulatory receptors.
-
- Modulation of antigen-presenting cell (APC) behaviour.
While Tregs are initially activated by antigen-specific mechanisms, their immune-modulatory effects are broadly non-specific, resulting in suppressive effects that extend to the tissue microenvironment. This can even promote the development of additional immunosuppressive cells, amplifying their therapeutic impact. Notably, infused Tregs may not need to persist long-term to achieve significant therapeutic effects, making them an attractive option for treating inflammatory conditions.
The Complex World of Treg Cell Therapy Manufacturing
The manufacturing of Treg therapies presents unique challenges, driven by the need for a highly pure population of rare and difficult-to-isolate cells. Although the starting material—such as leukapheresis or peripheral blood—is similar to that used in CAR-T manufacturing, the downstream processes for Tregs differ significantly due to their low cell numbers and small culture volumes. With most manufacturing platforms designed for larger cell numbers, Treg therapy introduces a host of complexities:
Key Manufacturing Challenges
-
- Small Numbers of Cells Tregs comprise a tiny subset of the T cell compartment. Manufacturing begins with processing large volumes of starting material, but once the Tregs are isolated, the culture volumes become very small. This makes process development difficult, as obtaining sufficient cells to test different culture conditions is challenging.
- Complex Isolation and Purification Achieving a pure Treg population requires multi-step processes, including de-bulking to remove unwanted cells and purification using magnetic beads and flow sorting. These steps are resource-intensive, both in cost and in time spent outside optimal culture conditions, which can impact cell health.
- Extended Periods of Culture Due to the low starting numbers of Tregs, prolonged culture periods are often needed to expand sufficient cells for therapeutic use. Maintaining Treg phenotype and function during this extended time is critical, but the longer culture periods increase the risks, including contamination and phenotypic drift.
- Technological Limitations Early-stage cell culture, often the most complex part of the process, frequently requires manual handling in open systems. This increases the risk of contamination and limits throughput, as most closed cell processing technologies are designed for larger cell numbers.
- Ensuring High Purity Contaminating effector T cells (Teffs) in the final product could exacerbate the very conditions Tregs are meant to treat. Strategies to ensure purity include careful monitoring, small molecule interventions (e.g., Rapamycin), and optimizing culture conditions to favour Tregs while limiting Teff growth.
The Promise of Treg Cell Therapy
Despite these challenges, Treg therapy offers transformative potential. It could provide better outcomes with fewer side effects than traditional immunosuppressive drugs. Unlike conventional medications requiring continuous administration, Treg therapy may offer long-lasting benefits after a single treatment.
The versatility of Tregs makes them potentially useful across a wide range of conditions. Beyond autoimmune diseases and transplantation, researchers are investigating their use in allergies, neurological disorders, and even cancer immunotherapy.
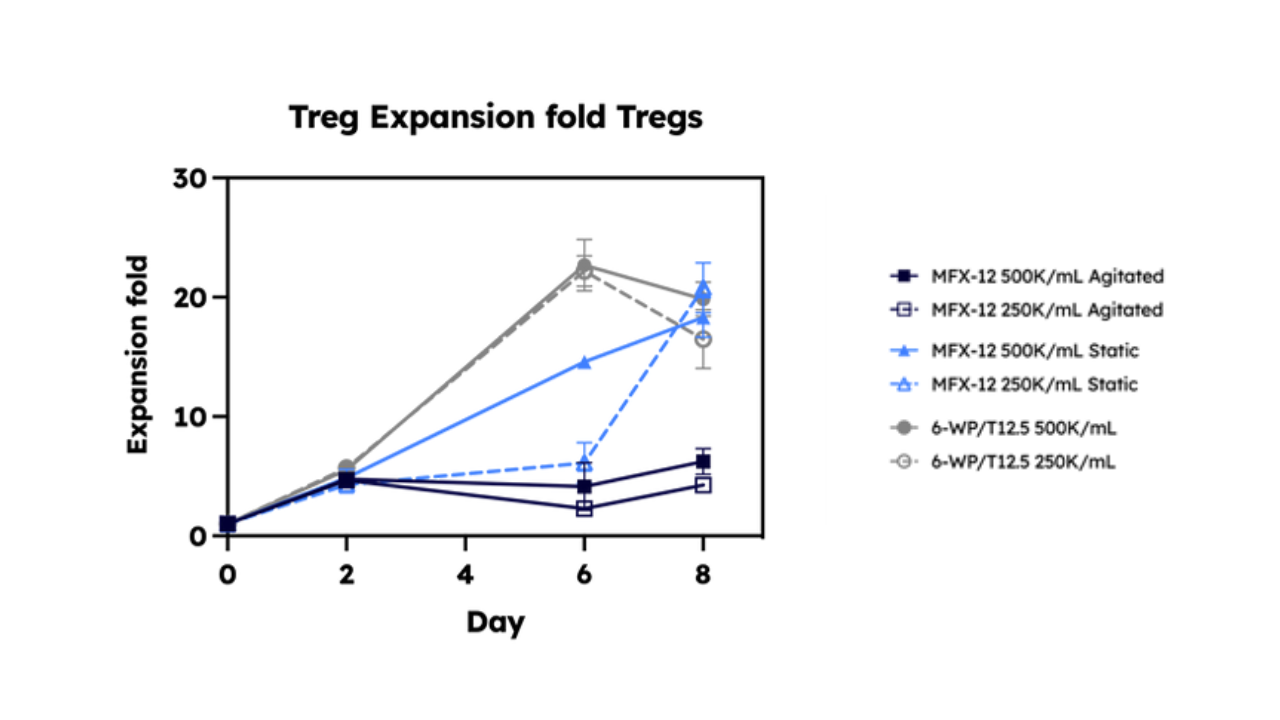
Looking Ahead: The Future of Treg Therapy
The field of Treg cell therapy is evolving rapidly, with new technologies and approaches emerging regularly. A significant shift is already underway, moving from polyclonal expanded cells to antigen-specific and engineered targeting. Advances in cell manufacturing, genetic engineering, and our understanding of immune regulation continue to unlock new possibilities. Allogeneic approaches where Tregs are derived from pluripotent cells in great numbers could remove some of the low volume cell processing and purification challenges.
While the journey from research to widespread clinical application is complex, the potential to fundamentally change how we treat immune disorders makes this pursuit worthwhile. As we continue to overcome technical challenges and gather clinical evidence, Treg cell therapy moves closer to fulfilling its promise as a revolutionary approach in immunotherapy.
Find out more about our recent project with AstraZeneca, where we expanded Tregs in the Cyto Engine, our novel, scalable bioreactor Download the poster
References
Treg cell-based therapies: challenges and perspectives Nat Rev Immunol. 2019 Dec 6;20(3):158–172.
Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review Yu Li, Cangang Zhang, Aimin Jiang, Anqi Lin, Zaoqu Liu, Xiangshu Cheng, Wanting Wang, Quan Cheng, Jian Zhang, Ting Wei & Peng Luo Journal of Translational Medicine volume 22, Article number: 293 (2024)
