From cell culture to cell therapy – the biggest challenges of growing cells ex vivo
During the development of the Cyto Engine platform we interviewed over 100 experts in cell culture and cell therapy manufacturing to understand what their biggest challenges were when culturing cells. Here’s what we found.
Cell culture is a fundamental technique in all biological research as well as in cell therapy production. It is the process of growing cells outside of their natural environment under controlled conditions and whilst it has been a cornerstone of biotechnology, pharmaceutical development, and academic research for over 100 years, the process remains complex and challenging to perform.
What did the 100 experts we interviewed highlight as being problematic? They stated contamination, reproducibility, scalability, integrated analytics, time and space constraints, and costs as pain points in their cell culture processes. These are all the problems we’ve set out to solve with the Cyto Engine.
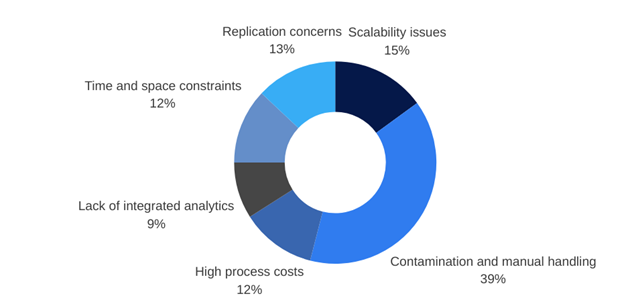
Cell culture pain points from the 100 experts we interviewed
1. Contamination: An Ever-Present Threat
Contamination remains one of the most significant challenges in cell culture. Experts across the board emphasised the need for stringent aseptic techniques and advanced detection methods. Scientists in Pharmaceutical R&D shared the following;
“Even with the most rigorous protocols, contamination can still occur. It’s not just about maintaining sterility; it’s about having the right tools to detect and eliminate contaminants early.”
“Current tools have too much human input.”
Contamination can come from various sources, including bacteria, fungi, and cross-contamination from other cell lines. It can compromise the integrity of the culture, leading to unreliable data and wasted resources. This is particularly concerning when manufacturing autologous cell therapies such as CAR-T, where each batch is patient-specific and losing batches could result in a significant delay in life saving treatments. The complexity of cell therapies often involves multiple stages of cell manipulation, increasing the risk of contamination. This is critical to avoid as there is no way of sterilising (e.g., filtering) the final product of a cell therapy (you would remove the cells you want to treat with!)
The need for ultra-clean environments, advanced contamination detection methods, and robust aseptic techniques is critical to ensure the safety and efficacy of these therapies. The industry is moving towards automation and less human touch on processes, but it will take time for meaningful changes to be enacted.
2. Reproducibility and Consistency
Another major challenge in cell culture is ensuring reproducibility and consistency across experiments. Experts from both CDMOs and biopharma companies highlighted that even slight variations in cell culture conditions can lead to significant differences in outcomes. This is particularly critical in drug development, where consistency is key to ensuring the efficacy and safety of a product.
“Reproducibility is not just a challenge; it’s a necessity.”
“We need more standardised protocols and better tools to monitor and control culture conditions in real-time.”
To address this, there is a growing demand for automated systems that can precisely control and monitor cell culture environments. These systems can help reduce human error, detect important trends early (especially in terms of donor variability), and ensure that conditions remain consistent across different batches, ultimately leading to more reliable results.
3. Scalability of Cell Culture Systems
Scalability is a crucial consideration for developers involved in large-scale production of biologics, particularly for those developing cell-based therapies. As one industry leader noted:
“Scaling up from a laboratory setting to commercial production is not just about increasing the volume. It requires careful consideration of how changes in scale can affect cell behaviour and product quality.”
A principal scientist at a biotech company highlights:
“There is lack of control and scaling in current systems, especially for adherent cells.”
The transition from small-scale to large-scale production often introduces new challenges such as: maintaining cell viability and phenotype, ensuring uniformity across large batches, and preventing contamination. There is a clear need for scalable bioreactors and culture systems that can accommodate these demands while maintaining high standards of quality and safety.
4. The Need for Advanced Monitoring and Analytical Tools
With the increasing complexity of cell-based therapies and growing demand for doses, the need for advanced monitoring and analytical tools is more pressing than ever. A biotech scientist emphasised the importance of real-time monitoring systems that can provide detailed insights into cell health, metabolism, and productivity:
“We need tools that can give us a real-time snapshot of what’s happening inside the culture. This not only helps in optimising culture conditions but also in predicting potential issues before they become critical.”
These tools are particularly important in the development of personalised medicine, where understanding the nuances of each individual batch can make the difference between success and failure.
5. High process costs
Another critical pain point in cell culture is the high cost of reagents (e.g., media, growth factors, and supplements), which significantly impacts budgets, particularly in long-term or large-scale experiments. As mentioned by one biotech scientist:
“We need systems with faster processing times and make use of cheaper reagents.”
A research institution commented:
“Working volumes and costs – when working with primary cells the cell number output can be a major challenge, it is not always feasible to seed 20+ T-flasks in order to get the desired number of cells as the reagent costs become incremental and the space is not always available.”
A university researcher from the same institution highlighted:
“Perfusion is still rare in academia because it drives up the cost of media.”
For large-scale applications like biomanufacturing or clinical trials these expenses can quickly skyrocket, limiting the feasibility of scaling up certain experiments or processes. Even routine cell culture maintenance becomes a costly affair when working with large volumes or sensitive cells that require expensive additives. One pharma manufacturing expert highlighted the need for:
“Shorter production time and higher success rate can help us decrease cost of goods.”
To address these high costs, researchers are exploring several strategies such as optimising media compositions to use fewer expensive components, or adopting serum-free media, which often lowers costs in the long term. Automation can further optimise reagent use, minimising waste and ensuring more efficient use of costly materials.
6. Time and space constraints
One of the biggest challenges is the large amount of time and space needed to cultivate cells, particularly when scaling up for clinical applications. Traditional T-flasks require significant incubator space, limiting how much can be done at any given time. In addition, manual processes in cell culture, such as media changes, cell counting, and passaging, are time-intensive and increase the risk of human error. The need for constant attention to ensure optimal growth conditions can be overwhelming.
One scientist at a research institution mentioned that:
“The amount of space it takes to grow the T-175 flasks takes 1 full incubator to make just one virus, which is also an issue as the amount of general consumables and costs e.g., media and pipettes, adds up a lot.”
Additionally, we heard about IPSC culture:
“A common drawback in the differentiation of cells is that it is a long [process] and the reproducibility is poor.”
Automation and miniaturised systems are increasingly being adopted to maximise space and optimise growth conditions. These systems enable researchers to grow more cells in less space without compromising efficiency.
Conclusion: A Collaborative Effort
Addressing these challenges requires a collaborative effort across the life sciences industry. From academia to big pharma, and from small biotech start-ups to global CDMOs, the need for innovation and collaboration has never been greater. By sharing knowledge and resources, the industry can develop the tools and techniques needed to overcome these hurdles, ultimately leading to more effective therapies and better outcomes for patients.
The insights we gathered from industry leaders underscore the importance of continued investment in research, technology, and infrastructure to address the complex challenges of cell culture. As we move forward, it is clear that the future of cell culture lies in a combination of advanced technology, rigorous standards, and a commitment to continuous improvement.
