Cutting the cost of CAR-Ts – Innovative strategies to make cell therapies more affordable
T cells are the most extensively studied cell type in the development of cell therapies, constituting 58% of active UK ATMP clinical trials (1). CAR-T (Chimeric Antigen Receptor T cell) therapy uses synthetically engineered receptors targeting a specific ligand in T cells to target and destroy tumour cells. Following three decades of development (2), CAR-T therapy has shown significant benefits in patients with B cell malignancies (3), and is being explored for broader indications such as autoimmune disease.
Currently, commercially available CAR-Ts are predominantly autologous therapies, being made for individual patients by extracting their T cells and modifying them in the lab. CAR genes code genetically engineered proteins that fuse an antigen recognition domain from a monoclonal antibody with the intracellular T cell signalling and costimulatory domains of the T cell (4). These proteins are then expressed on the surface of the T cells.
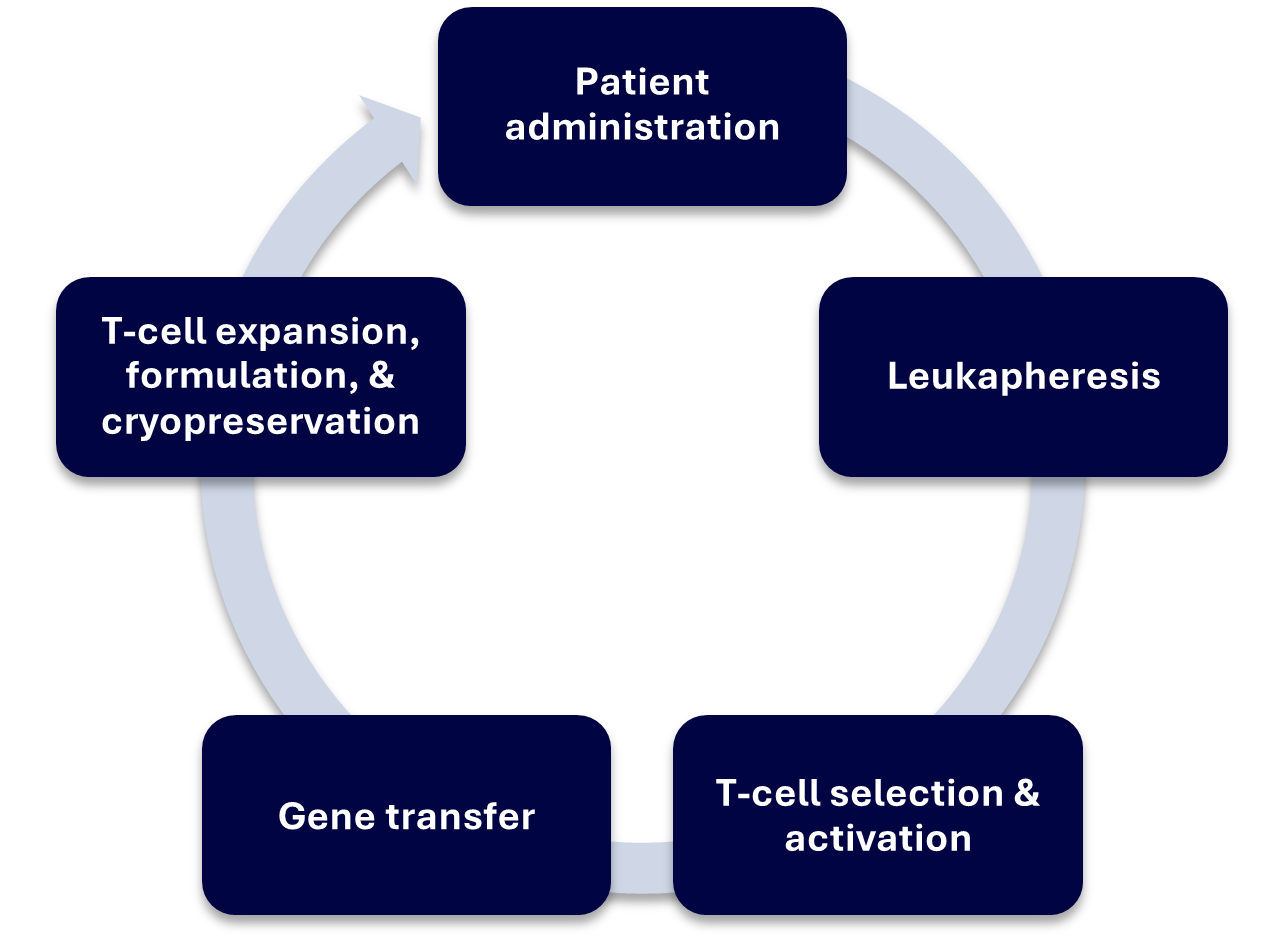
Major Steps in CAR-T Manufacturing Process (5,6)
CAR-T therapies typically take around 1-2 weeks to create, although exact processes can differ depending on the provider. Generally, the procedure involves:
- Leukapheresis
The patient’s T-cells are extracted using a separation process called leukapheresis, which collects cellular components from the blood. They are then sent to the manufacturing facility which may require them to be cryopreserved for transport.
- T-cell Selection & Activation
T cells are further purified from the leukapheresis and then activated using CD3/CD28 co-stimulatory antibodies or activation reagents and cytokines.
- Gene Transfer (Transduction or Transfection)
Viral vector is added to the T cells, or for non-viral approaches, electroporation or other novel techniques can be used to insert genes into the cell
- T-cell Expansion, Formulation & Cryopreservation
T cells are expanded to the required number of cells for a dose. Harvested cells are then cryopreserved for transport.
- Transport and Patient Administration
CAR-T cells are transported from a central manufacturing site to the hospital/medical centre and administered to the patient.
Despite the well-established protocols of production and high efficacy of these therapies, CAR-Ts only reach a limited number of patients. This is largely due to the cost, with the price of CAR-T therapies often reaching £400,000 per patient (8). One way to tackle these costs is by optimizing the current manufacturing process.
A variety of strategies are being investigated to reduce the expenses of CAR-T therapies. Some companies (1) are developing new technologies to automate parts of the manufacturing process, reducing labour costs and human error. A wider conversation within the cell and gene therapy (CGT) industry is looking at decentralised manufacturing, producing therapies at the point of care (9,10) or even in the patient themselves, removing the need for ex vivo manufacturing and reducing logistical costs.
The benefits and drawbacks of various CAR-T manufacturing strategies (7)
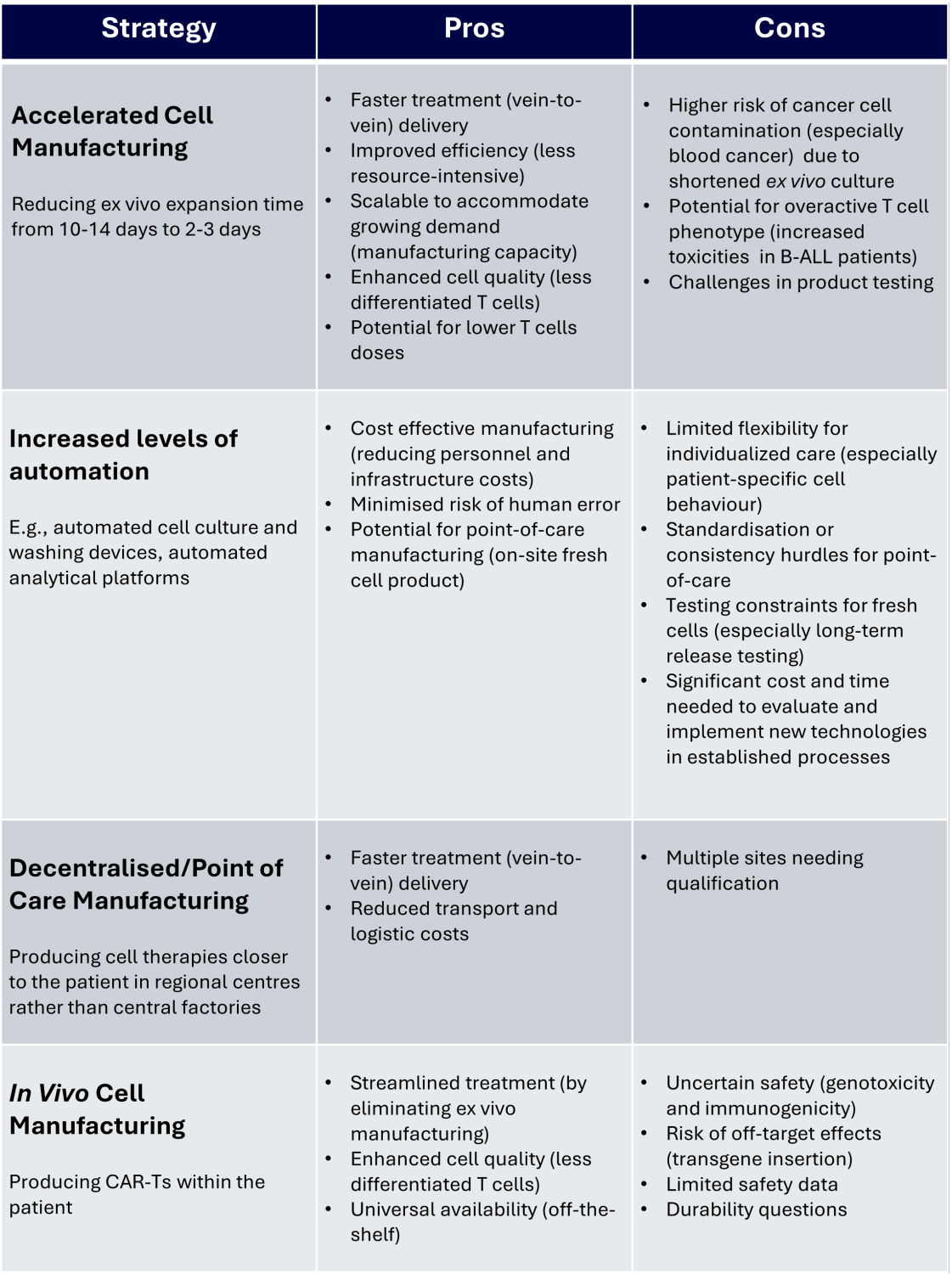
There is no single solution to make CAR-T therapies more affordable. However, the ongoing development of strategies to reduce manufacturing costs is a promising step to making these innovative and life-changing therapies available to everyone who needs them. worth waiting.
At MFX, we’re developing our Cyto Engine technologies to seamlessly scale up or down. This allows a greater understanding of the biology of CAR-Ts that enables faster process translation, and the ability to scale up cell numbers whilst maintaining the same microenvironment during manufacturing.
References:
- CATAPULT, Cell and Gene Therapy. Cell and gene therapy catapult. UK ATMP Clinical Trials Report. 2022.
- Charrot S & Hallam S. CAR-T cells: future perspectives. HemaSphere. 2019. 3:2.
- Xin T, Cheng L, Zhou C, Zhao Y, Hu Z & Wu X. In-vivo induced CAR-T cell for the potential breakthrough to overcome the barriers of current CAR-T cell therapy. Frontiers in Oncology. 2022. 12:809754.
- Levine BL, Miskin J, Wonnacott K & Keir C. Global manufacturing of CAR T cell therapy. Molecular Therapy: Methods & Clinical Development. 2017. 4: 92-101.
- Wang X & Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Molecular Therapy – Oncolytics. 2016. 3: 16015.
- Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol. 2018;53:164-181.
- Ayala Ceja M, Khericha M, Harris CM, Puig-Saus C, Chen YY. CAR-T cell manufacturing: Major process parameters and next-generation strategies. J Exp Med. 2024;221(2)
- Tumaini B, Lee DW, Lin T, Castiello L, Stroncek DF, Mackall C, et al. Simplified process for the production of anti-CD19-CAR engineered T cells. Cytotherapy. NIH Public Access; 2013; 15(11):1406.
- Lopes AG, Noel R, Sinclair A. Cost analysis of vein-to-vein CAR T-cell therapy: automated manufacturing and supply chain. Cell Gene Ther Insights. BioInsights Publishing, Ltd.; 2020; 6(3):487–510.
- Orentas RJ, Dropulić B, Lima M de. Place of care manufacturing of chimeric antigen receptor cells: Opportunities and challenges. Semin Hematol. W.B. Saunders; 2023; 60(1):20–4.
