MFX’s summer of students
MFX’s summer of students
Why investing time in the next generation helps give us new insights into our own technologies.
A common theme that has come up within the team at MFX is that when we were making decisions earlier on in our careers, the information about what options were available to us using our science background was often not entirely clear.
As we build out the company, we want to make sure that we are supporting the wider community by helping to build the talent pipeline entering STEM. We’re doing this by offering opportunities for visits, placements, and internships to interested students, as well as providing time for our team to engage in outreach activities. Check out our new people page to see what outreach activities our team have been up to.
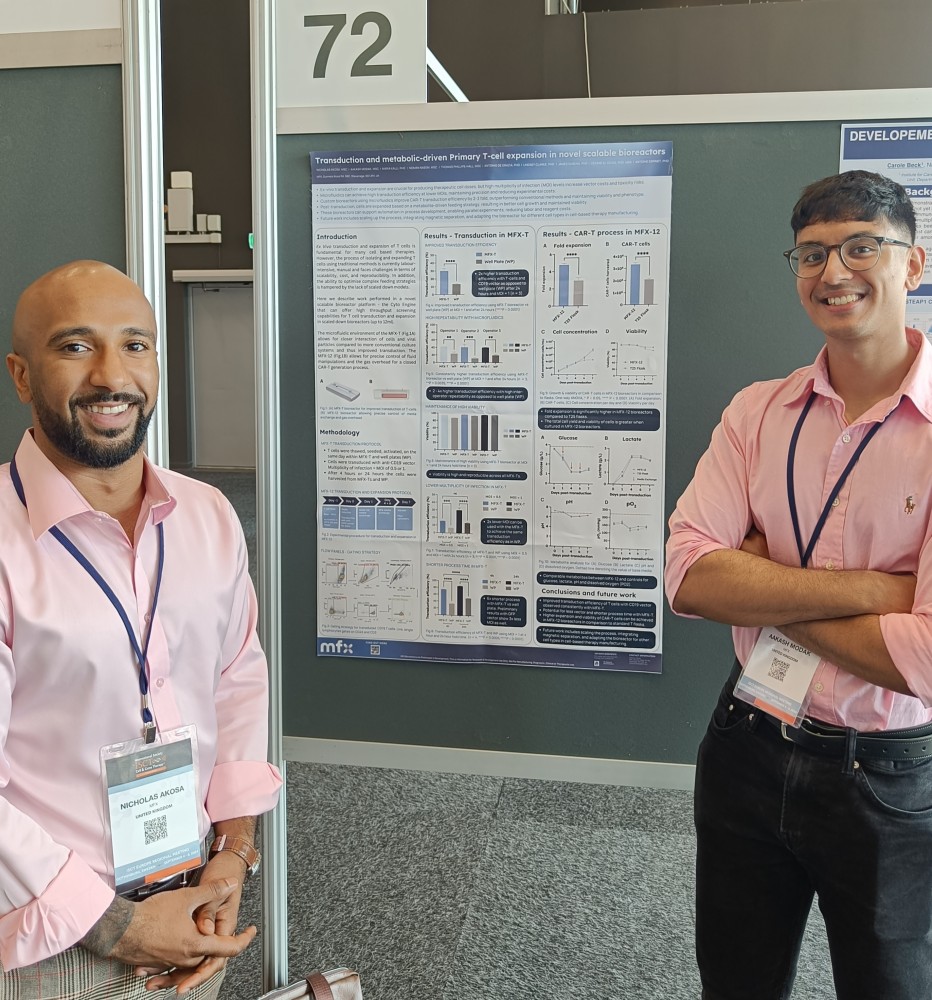
The first of our interns Aakash joined us at the start of the year, and we’re delighted to say he joined us as a full-time member of staff this summer in the R&D bioprocessing team! Here he is sharing one of our posters at ISCT.
Aakash and Nick with MFX’s poster on Transduction and metabolic-driven Primary T-cell expansion in novel scalable bioreactors at ISCT 2024
At around the same time we teamed up with UCL’s ‘Manufacture and Commercialisation of Stem Cell and Gene Therapies MSc’. We welcomed the whole cohort for a tour of our facilities and some pretty intense Q&A sessions with our team leads. We loved all the insightful questions from this fantastic group – talking through our technology and gaining insights is always helpful for us! We then welcomed Nabilla and San-Chi in our commercial and R&D teams respectively for 6-month placements.

Students from UCL’s Manufacture and Commercialisation of Stem Cell and Gene Therapies MSc

Nabilla and Maria at UCL’s Cell and Gene Therapy Research Project & Poster Session Day

Antonio, San-Chi, and James at UCL’s Cell and Gene Therapy Research Project & Poster Session Day
Whilst MSC level students and post graduate interns are well on their way to a fulfilling career in STEM, it’s apparent that access to companies like MFX earlier on in the decision-making process can influence students make the choice to stay focussed on STEM.
Over the summer we welcomed 3 work experience students for a week – 2 from local colleges and an undergraduate student home for the holidays – and provided tours to 2 students from local schools. Here’s what they had to say about the experience.
“I am so glad that I did this work experience, I really felt like it gave such an insight into the world of small start-ups, which I had previously known nothing about… It was also great being able to see all the range of roles required to make what they do happen….. I got to discover the jobs involved that are much less technical…[and] I got to see the more traditionally technical roles expected of scientists. Seeing all these people with different skill sets come together in a really innovative environment with a common goal was inspiring to watch.“
But it’s not all one-way benefits – the students really helped us in unexpected ways:
“When you‘re so deep in new tech development, it’s really easy to forget that not everyone is as familiar with what it is you are building as you are……” says Lindsey Clarke, PhD, Commercial VP. “so having to think about how we communicate what it is we are doing to non-experts and have the students pull us up on things like internal jargon has been really helpful – being able to distil the key benefits and features of the technologies and why we are building them is really important for both our sales and marketing efforts as well as fundraising.”
Conveniently, these extra pairs of hands were available during one of our bioreactor batch assembly runs – very much a stress test opportunity for our manufacturing SOPs and training! Fortunately we passed that with flying colours – so if you’re getting involved in the MFX early access trials this autumn, the likelihood is one of our work experience students helped manufacture your Prototype bioreactor!
Yes, it requires a significant investment of time to offer these opportunities to students. But the huge value it clearly brings makes it worthwhile, and who knows, perhaps we’ll see these familiar faces as future MFX employees! Good luck to all our students and interns with their future studies and career decisions. Maybe we’re biased, but STEM is where it’s at!